The Major Histocompatibility
Complex
The major histocompatibility complex (MHC) is another example of historical nomenclature in immunology. The name stems from work in the early 20th century on transplant experiments. It was demonstrated that transplants between species always failed, transplants between animals of the same species almost always failed, but transplants within one animal (almost) never failed. The molecules found to be important for this were named as tissue compatibility complexes. However the name MHC does not give any indication as to their normal function, namely presenting antigen to T-lymphocytes. The MHC is sometimes referred to as HLA (Human Leukocyte Antigens) but the terms are essentially synonymous.
One of the important differences between T- and B- lymphocytes is the type of antigen that they respond to. B-cells respond to native antigens on the surface of pathogenic organisms. T-lymphocytes were demonstrated to respond to short contiguous sequences of amino acids. These amino acids are derived from the invading organisms and then presented by the MHC molecules on the cell surface. Strictly, the T-cell receptor does not bind antigen, instead it binds the combination of antigen and MHC molecule.
The genes for the MHC molecules are found in one region of chromosome 6 that contains more than 100 genes.
The MHC molecules
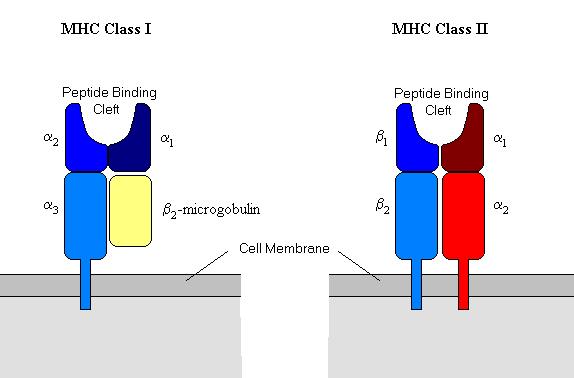
The MHC molecules are glycoproteins and are part of the immunoglobulin superfamily. There are two classes of MHC that have a slightly different function. They have very different amino acids sequences but very similar three-dimensional structure. They are known as MHC-I and MHC-II.
MHC-I is made up of four domains. Three of these domains are formed by one peptide - the a-chain (an MHC glycoprotein) - and the fourth domain is provided by a non-MHC encoded molecule named b2-microglobulin. The three domains of the a-chain are named a1, a2, and a3. a1 and a2 form the peptide binding cleft where the antigen is presented to the T-cell receptor and a3 contains a trans-membrane region that binds the whole molecule to the cell membrane. (See diagram below).
MHC-II is very similar and is made up of an a and a b chain, both of which are coded for by the MHC region in chromosome 6. Each chain has two domains; a1 and b1 form the peptide binding cleft and both a2 and b2 are membrane bound.
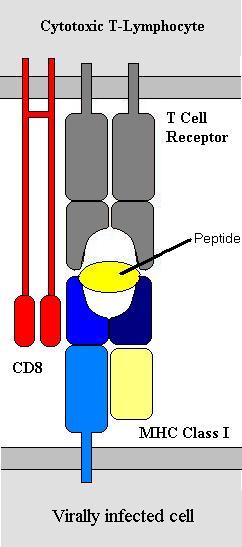
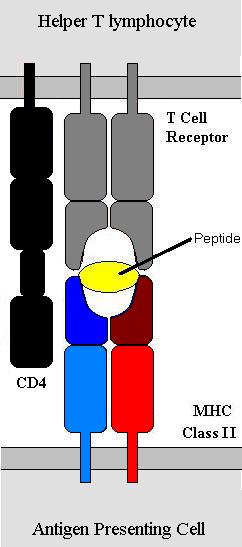
MHC-I is found on all nucleated cells and is central to anti-viral immunity. MHC-II, by contrast is found on a small number of specialist cells known collectively as antigen-presenting cells (APCs). Antigen presenting cells include macrophages, B-lymphocytes, cytotoxic T-lymphocytes and several others. Cytotoxic T-cells express the molecule CD8 with the T-cell receptor. CD8 binds to MHC-I and not MHC-II. For this reason Cytotoxic T-cells bind to MHC-I. T-helper cells do not express CD8 but do express CD4 which works in a similar but contrasting way – it binds to MHC-II only and thus T-helper cells only bind to MHC-II. Hence the molecules CD4 and CD8 are very important in defining the function of different T-lymphocytes. This is why T-helper cells are also know as CD4 cells and cytotoxic T-cells as CD8 cells.
 =============
=============
Antigen Processing
The peptide antigens are small (~6-8 amino acids) and non-covalently bonded to the MHC molecules. Hence they use van-der-Waals interactions, ionic charge etc. to bind. Proteins are broken down to peptides continuously by mechanisms within the cell. These peptides are then bound to MHC molecules that then move to the cell surface. The process of antigen processing is slightly different between MHC-I and MHC-II but the principal is the same that peptide antigens are produced that are then expressed on the cell surface. In a non-infected cell the antigens presented by MHC-I will be self-antigens and the T-lymphocytes will not bind to the cell. If however the cell is infected then peptides presented will be viral peptides and the T-cell receptor will bind and initiate killing of the infected cell. In a similar way APCs such as B-lymphocytes will process antigen and present it on their MHC-II molecules. The binding of the B-cell to a T-helper cell induces the T-helper cell to release certain cytokines that trigger various parts of the immune response.
MHC Genetics
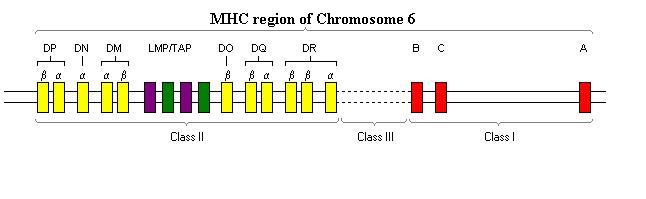
The genetics of the MHC are very unusual and have been intensely studied. Unusually, instead of having two genes for the MHC-I a-chain, each individual has 6 (3 on each chromosome). These are known as HLA-A, -B and –C. They are all co-expressed, so that each individual has 6 different MHC molecules on each cell. Similarly the MHC-II has multiple genes; they are DP, DQ and DR. Each chromosome 6 has a DPa and a DPb. Similarly, each has a DQa and a DQb as well as a DRa and one or two DPb genes. This concept (called polygeny) means that each individual expresses a range of MHC molecules.
Polygeny is functionally very significant as each MHC molecule binds a different range of peptides. MHC-I molecules present viral antigen to cytotoxic T-cells, enabling the virally infected cell to be killed. It is very possible that if each cell had only one MHC type, a virus could evolve proteins that, when broken down by the cell's machinery formed peptides that could not be presented by that MHC molecule. Such a virus would be lethal as it would be 'invisible' to the immune system. By expressing 6 different MHC-I molecules the cell is able to present to T-cells a very wide array of antigens. For example, some MHC molecules present positively charged peptides; others, negatively charged and others, non-charged peptides.
An inevitable question that arises at this point is why only 6 MHC molecules per cell – why not 10, 20, 100? The answer is thought to be self-tolerance. A cytotoxic T-cell that binds to an MHC-A molecule (for example) plus foreign antigen may bind to a MHC-B molecule plus a self-antigen due to structural similarities. Any T-cells that do respond to self-antigens are eliminated and thus if there were a large number of MHC molecules, the repertoire of T-cells would be dramatically reduced and thus the immune system would not be able to respond to these infections. It is generally thought that the number of molecules of MHC expressed is a balance – enabling as many antigens as possible to be presented whilst maintaining as large a repertoire of T-cells as possible. This cross-reactivity of T-cells is the mechanism of transplant rejection. T-cells respond to the MHC molecules that are non-self as though they were presenting foreign antigen. For this reason ‘transplant matching’ is about matching the MHC types between the donor and recipient. The reason that transplants still fail is that the matching is imprecise and even if two individuals have apparently the same MHC types, they may still differ because the MHC is an incredibly variable region of the human genome.
The concept of polymorphism of the human genome is an important part of immunity on the species level. There are 25 known variants of the HLA-DQb locus and 16 of the DQa, meaning that there are literally hundreds of different DQ MHC-II molecules in the human population. The same principal applies to each of the MHC genes (except that DRa has only one known allele). This variability means that whilst some pathogens may be lethal to the individual, it is much more difficult for the said pathogen to be able to evade the immune system of entire populations.
As well as the MHC genes, included in this region of chromosome 6 are several other genes (and also pseudogenes). Examples include the genes for complement proteins C4, C2 and Factor B, the cytokines tumour necrosis factor a and b and TAP genes. The TAP genes (Transporters associated with Antigen Processing) encode for the proteins TAP-1 and TAP-2 that function in antigen processing, breaking down intracellular proteins to the small peptides that MHC molecules present.
Fighting Viral Infections
The MHC plays a central role in fighting viral infections: MHC-I on all cells presents peptides from intracellular proteins that necessarily will include viral peptides if the cell is infected. It is this combination of MHC molecule and viral peptide that cytotoxic T-cells respond to.
The MHC-II molecule has a different role and is important in fighting both bacterial and viral infections. Its function is discussed more fully in the section on T-helper cells.