T-Helper Cells and HIV
There is no evidence that T-helper cells have (direct) means of killing pathogen. However it would be wrong to deduce therefore that they play only a minor role in the immune system. The pathogenesis of HIV demonstrates the fundamental importance of the T-helper cell in the functioning of the immune system. Both HIV-1 and HIV-2 primarily infect T-helper cells and as a consequence the patient suffers from a dramatic drop in their number of T-helper cells. This results in a profound immunocompromise and thus clearly the T-helper cell, despite its lack of direct killing is vital to fighting infections. Conversely, its lack of direct killing is perhaps why it is so important as it coordinates both the cell-mediated and the humoral arms of the immune system. From a basic understanding of the function of T-helper cells it is possible to deduce what effects HIV infection will have and conversely HIV infection demonstrates the role of T-helper cells, hence this section discusses both these topics together. In HIV medicine T-helper cells are almost always referred to as CD4 cells. The terms T-helper cells and CD4 cells are synonymous. As described in the section on the MHC, the CD4 molecule is central to the functioning of the T-helper cell, but it is also the reason that HIV preferentially infects T-helper cells, as the CD4 molecule is the ‘HIV receptor’. HIV binds to CD4 as the first stage of entry into the cell.
TH1 and TH2
T-helper cells are generally subdivided into types 1 and 2 (TH1 and TH2 respectively). This division reflects a functional difference between these two cell types; TH1 cells are part of the cell mediated immune system and TH2 cells, the humoral system. TH1 cells interact with cytotoxic T cells and with macrophages. The cytokines they release are important for activating the macrophage. This is most important for killing intracellular bacteria such as listeria and tuberculosis that infect the macrophage itself.
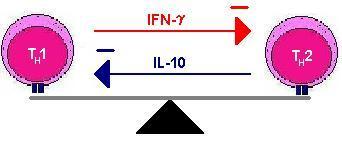
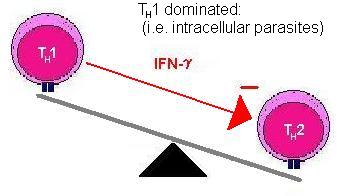
The two types of helper cells also regulate each other. TH1 cells produce cytokines that suppress TH2 cells and vice versa, hence an immune response is often said to be either TH1 or TH2 dominant. (Move mouse over second image).
This polarisation is seen with some infections and can be important in enabling the body to fight the infection. It is thought that if the immune response is not TH1 dominant to tuberculosis infection then the infection will become chronic.
The following table summarizes the differences in function between the two sub-types of TH cell. These functional differences are conferred by the cytokines that the cells produce.
|
TH1 Cells |
TH2 Cells |
||
|
IL-3 |
Growth
of progenitor haemopoetic stem cells |
||
|
GM-CSF |
Growth of
myeloid progenitor cell and consequently all myeloid cells |
||
|
IL-2 |
T-cell
growth |
IL-4 |
B-cell
activation and growth IgE
isotype switching IgG isotype
switching (IgG1 not IgG3) Induction
of MHC-II expression T-cell
growth |
|
IFN-g |
Macrophage
activation Induction
of MHC-II expression Inhibition
of TH2 cells Induction
of Tc cells |
IL-5 |
Eosinophil
growth IgA
isotype switch |
|
TNF-a |
Macrophage
activation |
Il-6 |
B-cell
growth Acute
phase protein release |
|
TNF-b |
Cytotoxicity Macrophage
activation Neutrophil
activation |
Il-10 |
Inhibits
macrophage activation Inhibits
TH1 cells |
|
|
|
TGF-b |
Inhibits
macrophage activation |
It is thought that na´ve TH cells are not committed to becoming either TH1 or TH2 cells and this decision is made by whichever APC they bind to. Na´ve TH cells are thus sometimes referred to as TH0 cells. If a TH0 cell binds to a macrophage it will become a TH1 cell and if it binds to a B-cell it will become a TH2 cell.
This division of TH cells into types 1 and 2 is well
established and is descriptive. However some have suggested that it is slightly
too simplistic and that there is really a spectrum of cells that secrete a
range of cytokines. Whist this may be true, for the purposes of understanding
the function of TH cells it is helpful to consider them as two
discrete cell types.
Humoral Immunity – B-lymphocytes and T-helper cell interactions
B-lymphocyte activity is greatly enhanced by TH2 cells. The primary signal for B-cells to expand and differentiate is the binding of antigen by the B-cell receptor. However the binding of the T-helper cell to the B-cell is also important. After binding to antigen B-cells have the ability to take up antigen and process it for presentation by MHC-II molecules. In this way B-cells are acting as antigen presenting cells.
The binding of the TH2 cell triggers clonal expansion and differentiation of the B-cell in two ways. Firstly when the TcR binds to the MHC this causes the expression of various surface molecules such as the molecule CD40L (CD40 ligand). The CD40 ligand binds to CD40 which is expressed by the B-cell and this is an important signal to the B-cell to grow. Secondly, TH2 cells, in response to the binding of TcR to MHC, release cytokines such as IL-4, IL-5 and IL-6 that act on the B-cell. IL-4 is important in inducing isotype switching of the B-cell from IgM to IgG. This, however, is not a simple relationship with IL-4 inhibiting some sub-types of IgG and inducing others. IL-5 induces isotype switching to IgA. Importantly, the B-cell - TH2 cell interaction is vital for isotype switching and for the production of memory B-cells. The importance of this interaction is discussed further in the section on vaccination.
CMI – Macrophage and T-helper cell interactions – Tuberculosis
It is in tuberculosis and similar pathogens that the importance of TH cells to cell-mediated immunity is most clearly seen. The Mycobacteria that cause tuberculosis are taken up by macrophages like any other bacteria but they are then able to grow within the phagosome and are not killed by non-activated macrophages. This is because they are able to prevent the fusing of the phagosome and the lysosome. This becomes an ideal location for bacterial growth because it is entirely protected from the immune system within a phagosome. The mechanisms that prevent fusion of the phagosome and lysosome are quite complex and vary between different bacteria that are able to do the same thing. However activation of the macrophage by TH1 cells leads to the killing of the bacteria and hence the TH1 cell is critical to tuberculosis resistance.
HIV infection and T-helper Cells
It is estimated that more than 37 million people are currently living with HIV. More than 70% of these are in sub-Saharan Africa.1 The clinical presentation of HIV varies widely: a patient may be asymptomatic and present for testing due to a known exposure or, conversely may have what is terms an AIDS-defining illness such as Karposi’s Sarcoma.
Virologically, HIV is a retrovirus which means it is an RNA virus that makes a DNA copy of its genome and then integrates into the host’s genome. Then it uses all of the host cell’s cellular machinery to express the viral proteins. There are two reasons why HIV is a devastating infection. Firstly, it has incredible antigenic diversity that enables it to evade the adaptive response of the immune system. Secondly, its primary cell tropism (the cell it infects preferentially) is the TH cell. As a result of this infection the TH cells are killed, though it is not entirely clear whether HIV kills the cell directly or whether TC cells perform the killing. The long asymptomatic period of HIV is a result of the body's ability to replace the lost TH cells. However at some point this replenishment fails and then the patient becomes symptomatic. CD4 levels are measured for patients who are HIV+ and there is a strong correlation between CD4 levels and the types of diseases the patient will contract.
Patients do not die of HIV itself. As a result of HIV infection, a patient suffers immense immunocompromise and they die from secondary infections. Given how vital TH1 cells are in TB, it is not surprising that there is a great deal of synergy between HIV and TB. Often the tuberculosis has been present and dormant for years and is reactivated when the CD4 levels fall.
HIV demonstrates how incredibly effective the immune system is at killing potential pathogens. Many organisms that are never normally infective are the cause of life-threatening disease to a HIV infected individual. The fungal infection Pneumocytis carinii pneumonia is only seen in HIV+ patients. Similarly parasitic meningitis is unheard-of in immunocompetent individual. Furthermore, whilst oral candidiasis is seen in many people (such as those on inhaled steroids), oesophageal candidiasis is very suggestive of HIV infection.