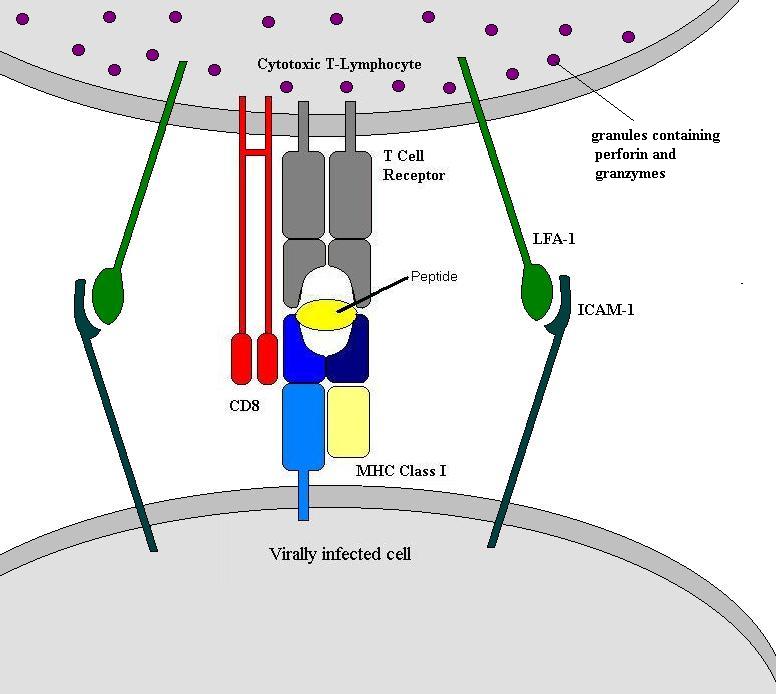
Cytotoxic T-Lymphocytes
All T-cells express a T-cell receptor - that
is what makes them T-lymphocytes. The T-cell receptor is analogous to the
immunoglobulin molecule. In this section, the development of the T-cell receptor
is described mostly in terms of its similarity to and differences from the
immunoglobulin production of a B-lymphocyte. Therefore to understand this
section, it is necessary to first read the section on antibodies.
The T-cell Receptor
The T-cell receptor is made up of an a-chain and a b-chain. These are considered analogous to the light and heavy chains of the immunoglobulin molecule. Unlike the immunoglobulin, the T-cell receptor is monovalent, having only one of each chain (c.f. two light and two heavy chains in each immunoglobulin molecule). Each of the chains has a variable and a conserved region. The variable region makes the antigen binding site and is produced by the same mechanisms by which immunoglobulin genes are produced.
The a-chain has between 70 and 80 V segments, 61 J-segments and one constant segment. The b-chain has 52 variable segments, followed by one D segment, 6 J-segments and one C-segment. This is then repeated with another D-segment, 7 J-segments and one constant region.
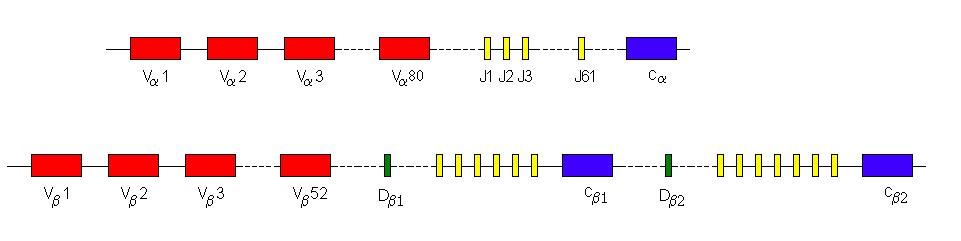
The a-chain rearrangement joins any one of the 70-80 variable segments with anyone of the 61 J segments. The b-chain rearrangement is slightly different as the Db1 joins to any one of the six Jb1 segments or the Db2 joins to any one of the seven Jb2 segments. This newly-formed DJ-segment then joins to any one of the 52 V-segments. Therefore on the basis of segment selection only, there are 70 x 61 = 4270 a-chain permutations and 13 x 52 = 676 b-chains. There are 3100020 (~3x106) possible different T-cell receptors on the basis of random selection of segments alone. In the same way as immunoglobulin rearrangement, this number is vastly increased by joint diversity using N- and P- nucleotides. The D-segments in the TcR genes are mostly able to be read in all three reading frames. This has two advantages. Firstly, it means that when the addition of P- and N-nucleotides changes the reading frame a functional gene product can still be produced. Secondly it means that each D-segment has three different possible amino-acid sequences and thus there are effectively 6 different D-segments to choose from.
The one process that occurs in immunoglobulin genes that is not replicated in the T-cell receptor genes is somatic hypermutation.
T-cell Development
All lymphocytes are derived from a common lymphoid progenitor cell. In the case of T-lymphocytes these cells migrate from the bone marrow to the thymus. The thymus is a small organ that is located in the mediastinum very close to the heart. It was thought that the thymus is only active before birth and it regresses and shrinks from birth onwards. However, there is now evidence that it retains some function even into adulthood.
The random selection of gene segments and the introduction of N- and P-nucleotides produces a vast array of T-cell receptors. The function of the thymus is to produce a useful repertoire of T-lymphocytes. Many of the T-cell receptors produced will not bind to MHC and are therefore useless. Many produced will bind to MHC presenting self antigens and therefore will be dangerous – able to produce an autoimmune response. Therefore T-cells have to undergo both a positive and a negative selection. The positive to bind MHC and the negative to not bind MHC+self antigens. Approximately 50 million T lymphocytes enter the thymus in the mouse and only around 2 million are released into the blood stream. Therefore there is clearly a very high level of wastage. The T-cells that fail to be selected die by apoptosis and are ‘mopped up’ by thymic macrophages. By these mechanisms mature T-cells are produced that are able to respond to foreign antigens presented on MHC molecules and those that bind self-antigens are eliminated. Some self-antigens are not expressed in the thymus (e.g. insulin and antigens not expressed until puberty) therefore some of the T-cells released from the thymus are potentially self-reactive. It is thought that a mechanism called peripheral tolerance or anergy does not kill these cells but is able to inactivate them.
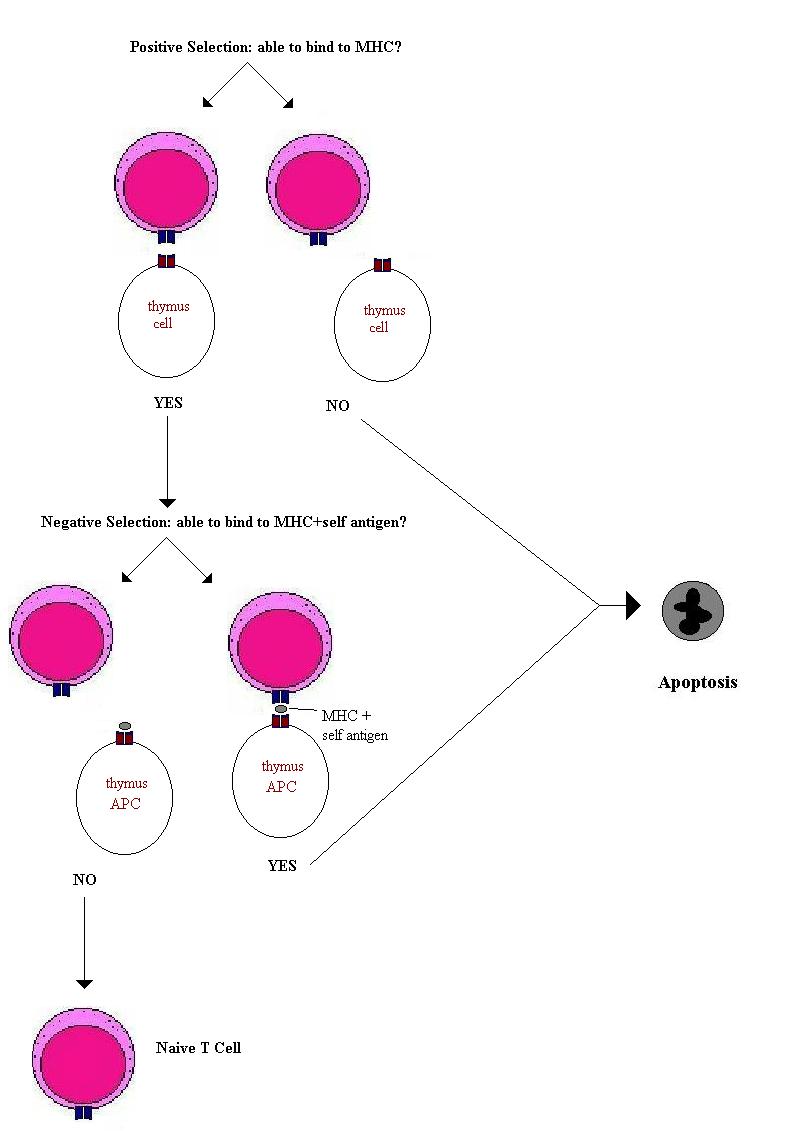
This repertoire selection process in the thymus applies to both T-helper cells and to cytotoxic T-cells. It produces T-lymphocytes with functioning but non-self reactive T-cell receptors. However the T-helper cells and T-cytotoxic cells express many different molecules on their surfaces that confer their different functions. First among these is the CD8 or CD4 molecule that defines which type of MHC molecule the T-cell receptor will bind to.
Killing Virally Infected Cells: Cytotoxic T-Lymphocytes
The virally infected cell will be expressing MHC molecules with viral peptides in their peptide clefts. This is bound by the T-cell receptor and CD8 molecule and as a consequence, the infected cell is killed. Unsurprisingly it is actually slightly more complex than this and involves several different molecules.
Another mechanism is the Fas and Fas-ligand interaction. Fas is a molecule expressed on some cells. When Fas is bound to the Fas ligand it induces apoptosis in that cell. The Fas ligand is constitutively expressed by cytotoxic T-cells. Activated T-cells are able to induce the expression of Fas on infected cells. The binding then occurs between the T-cell receptor and MHC molecule and between the Fas ligand and Fas molecule.
As well as killing cells directly, cytotoxic cells can act indirectly via cytokines. Interferon gamma (IFN-g) and tumour necrosis factors alpha and beta (TNF-a and -b) are examples of this. IFN-g increases the expression of MHC molecules by inhibiting viral replication and activating macrophages. TNF-a and -b are inducers of inflammation and are directly cytotoxic.
Once a cytotoxic T-cell has killed one cell it moves on and kills the other cells expressing the same antigen. And, because the cytotoxic molecules are pre-formed and stored in granules there is no delay for de novo synthesis. Thus cytotoxic T-cells are very efficient killers of infected cells.