Natural Killer Cells
Natural killer cells
are part of the innate immune system and are important in fighting intra-cellular
pathogens. In mouse experiments, a mouse lacking both B and T cells can resist
Listeria infection (which is caused by an intracellular bacteria) for a number
of weeks but if the NK cells are depleted it will die from Listeria infection
very quickly. Although this ability to kill viruses without antigen specificity
is impressive it is not likely to be physiologically important in humans.
An absence of NK cells is very rare but those individuals who do lack NK cells
have essentially no immunocompromise, only being more susceptible to the early
stages of herpes virus infections.
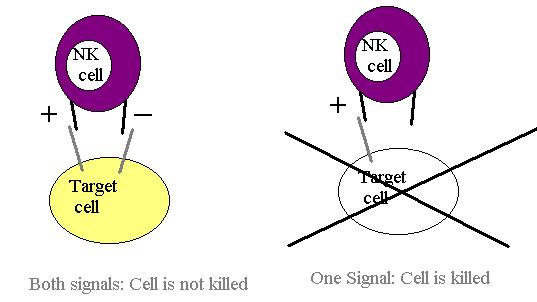
Natural Killer cells are lymphocytes that lack antigen specificity and thus they must rely on some other mechanism to kill virally infected cells. This mechanism depends on the presence of two receptors on the surface of the NK cell. These are NKR-P1 and Ly49. NKR-P1 binds to host cell carbohydrates, triggering the NK cell to kill the host cell to which it is bound. The Ly49 molecule binds to the MHC-I molecule, thus inhibiting the killing activity of the NK cell. If both signals are activated simultaneously the inhibitory one is dominant and so the cell is not killed.
Many viruses inhibit
the production or expression of the MHC-I molecule. This is entirely logical
as the killing of the virus by cytotoxic T-cells depends upon the MHC-I molecule
presenting viral antigen. One example of this is the adenovirus E3 that produces
a glycoprotein that sequesters MHC-I molecules in the endoplasmic reticulum
and thus prevents their expression on the cell surface. This is where NK cells
function. In the absence of the MHC molecule, there is no signal to override
that of the NKR-P1 molecule and hence the killing action of the NK cell is
activated and the cell not expressing MHC-I will be killed. It is possible
that NK cells act against tumour cells in the same way, by inducing killing
in response to the down-regulation of MHC molecules.
Interferon a and b are produced by many cells in response to viral infection and
have two important actions. Firstly, they enhance the activity of NK cells
by between 20 and 100 fold. The cytokine IL-12 (also known as NK cell-activating
factor) has a similar and synergistic effect. Secondly, they increase the expression of MHC-I thereby enhancing the activity
of cytotoxic T-cells.
The interferon levels
rise immediately after infection. The killing of virally infected cells by
T-lymphocytes takes 2-3 days to commence and does not reach its peak until
7-8 days after the initial infection. Natural Killer Cells fill this gap and
kill infected cells in the first 5-6 days of infection before the cytotoxic
T-cells are active.
NK cells also interact
with antibodies. Antibodies are generally not important in fighting viral
infections. Most of the killing of viruses is done by the cytotoxic T-cells.
However antibodies do act in two ways. The first is that antibodies will bind
to the surface of a virus in the body. In some cases the surface antigen that
the antibody binds will be vital for the viral entry into cells and thus the
antibody will inactive the virus and prevent it from infecting cells. The
second method is known as antibody-dependent cell-mediated cytotoxicity.
In some cases an infected cell will express on its surface viral antigens
that are recognised by antibodies. The binding of these antibodies triggers
NK cell to kill the infected cell because the NK cells have Fc
receptors.