Antibodies
It is estimated that each individual has an
incredible 1011 different antibodies. Each of these antibodies is
produced by a different B-lymphocyte. If this lymphocyte is activated it will
divide and differentiate into memory B cells and plasma cells. The memory cells
are long lived cells that enable a faster immune response to the same antigen
in the future, thereby providing immunity to that particular infection.
Concurrently, plasma cells produce large numbers of antibodies to fight the
current infection.
Antibodies
and Immunoglobulins
These two terms are often used synonymously and in most
cases they do mean the same thing. However they are not strictly identical
terms. Antibodies are immunoglobulins and the B-lymphocyte receptor is also an
immunoglobulin. Antibodies are free in plasma whilst the B-lymphocyte receptor
is membrane bound. Another important term is the Immunoglobulin superfamily.
Immunoglobulins are glycoproteins; that is, they are proteins that have sugar
groups bound to them. Within immunology there are several other glycoproteins
that have a great deal of homology with immunoglobulins (such as the
T-lymphocyte receptor) and hence they are referred to as members of the
immunoglobulin superfamily.
Structure
of Immunoglobulins
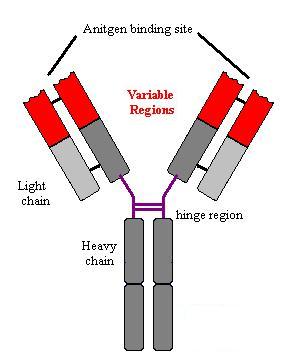
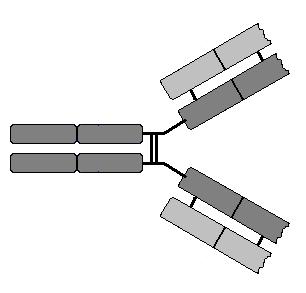
Immunoglobulins are made up of two identical heavy chains
and two identical light chains. The heavy chains are coded for by one gene on
chromosome 14. There are two types of light chain – known as l and k; the
genes are on chromosomes 22 and 2 respectively. There is no functional
difference between l and k chains. Each
immunoglobulin has two antigen binding sites. These sites are formed from the
variable domains of the light and heavy chains together. The light chains have
one variable region and one constant region. The heavy chains have one variable
region and three or four constant regions. There are five different heavy
chains which are known by the Greek letters m, g, a, e, and d. The
picture shows the basic common structure of immunglobulin molecules. (Move
mouse over image)
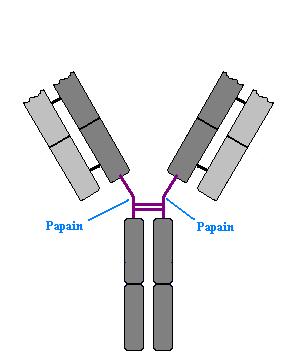
One of the earlier studies done on immunoglobulins was treatment
with proteases. When treated with papain, immunoglobulins are cleaved just
below the hinge region producing two different fragments.. These are know as
Fab (Fragment Antigen Binding) and Fc (Fragment crystallisable).
This is important as the crystallisable fragment contains the tail of the
constant regions of the immunoglobulin. Many cells, such as macrophages, have
receptors that bind to the constant region of antibodies. These receptors are
known as Fc receptors (FcR). (Move mouse over image)
Classes
of Immunoglobulin
There are five classes of immunoglobulins:
IgM, IgG, IgA, IgE and IgD. The class of immunoglobulin is defined by the heavy
chain; i.e. IgM has m heavy chains, IgG, g heavy chains etc. Each of these classes of
immunoglobulin have different properties.
|
Immunoglobulin |
Heavy Chain |
Molecular weight
(kDa) |
Serum |
Complement
Activation |
Placental
Transfer |
Phagocyte
Binding |
Mast Cell/
Basophil binding |
||
|
Level (mg ml-1) |
Half live
(days) |
||||||||
IgG
|
IgG1 |
g1 |
146 |
9 |
21 |
++ |
+++ |
+ |
|
|
IgG2 |
g2 |
146 |
3 |
20 |
+ |
+ |
|
|
|
|
IgG3 |
g3 |
165 |
1 |
7 |
+++ |
++ |
+ |
|
|
|
IgG4 |
g4 |
146 |
0.5 |
21 |
|
+ |
|
|
|
IgM
|
m |
970 |
1.5 |
10 |
+++ |
|
|
|
|
IgA
|
IgA1
|
a1 |
160 |
3.0 |
6 |
|
|
+ |
|
IgA2
|
a2 |
160 |
0.5 |
6 |
|
|
+ |
|
|
IgE
|
e |
188 |
5x10-5 |
2 |
|
|
+ |
+++ |
|
IgD
|
d |
184 |
0.03 |
3 |
|
|
|
|
|
IgM
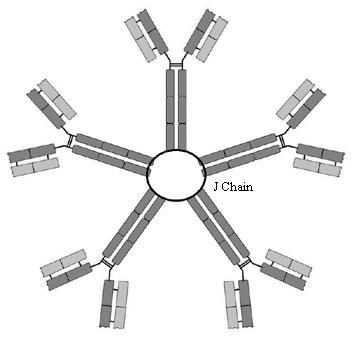
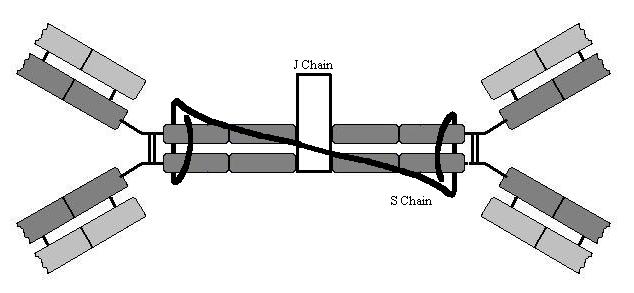
In plasma IgM is a pentamer (held together by
a protein known as a ‘J-chain’(J for joining)) and thus it can cross link
several antigens. This cross-linking causes precipitation of pathogens and
toxins in plasma. IgM (in a monomer form) is also the B-lymphocyte receptor.
The difference between secreted IgM and the B-lymphocyte receptor is one domain
on the protein; either a secretory component that enables it to be released
into plasma or a membrane-bound component that binds the immunoglobulin to the
cell membrane. This is achieved by alternate splicing of the mRNA to either
contain a secretory region or a trans-membrane region on the tail of the
molecule. Secreted IgM is the first antibody produced by B-lymphocytes.
IgG
IgG is the most abundant in plasma. It is
able to activate complement and is a powerful opsonin. It also crosses the
placenta and thus provides passive immunity to the neonate.
IgA
IgA is primarily involved in mucosal
immunity. Both IgA1 and IgA2 are dimers and in addition they contain a J chain
similar to IgM and an S chain (S for secretory) that prevents the antibody from
being broken down by the host proteases found on epithelial surfaces. IgA is
the type of antibody found in breast milk and thus breast feeding introduces
these antibodies directly to the epithelial surface of the gastrointestinal
tract of the infant.
IgE
The precise function of IgE is not well understood.
IgE is thought to be important in allergic reactions because it is very potent
in activating mast cells and basophils and hence induces histamine release
which is known to be a major mechanism of allergy. IgE also appears to play an
important role in fighting parasitic infections along with eosinophils. Large
worms cannot be ingested by phagocytes and so they are killed by a process
known as exocytosis. The invading worm becomes coated with IgE which then binds
to the Fce receptor on
the eosinophils that release toxic granules onto the parasite.
IgD
IgD is found at low levels in plasma but its function
is unclear. Mice deficient in IgD show no immunocompromise and thus it is not
thought to be important. In na´ve B cells it is also co-expressed as a
membrane-bound immunoglobulin with IgM.
Immunoglobulin Production.
The ability to produce an incredible array of
immunoglobulins that bind to foreign antigens is key to the ability of the
immune system to mount an adaptive response to infection. This diversity is
produced by a number of mechanisms.
Lymphocytes break what is known as somatic
theory. Somatic theory states that every cell within a complex organism has
the same genetic material within it and the differences between the cells are
determined by which genes are expressed. Lymphocytes, uniquely, are able to
rearrange their genome. B-lymphocytes do this with the immunoglobulin genes and
T-lymphocytes with the T-lymphocyte receptor genes. The mechanisms used are
essentially the same for both with a few important differences. Genetic
rearrangement is very well controlled within the cell. This is vital as
otherwise it would be extremely carcinogenic. The genes responsible for this
are know as RAG-1 and RAG-2. (Recombination Activating Genes).
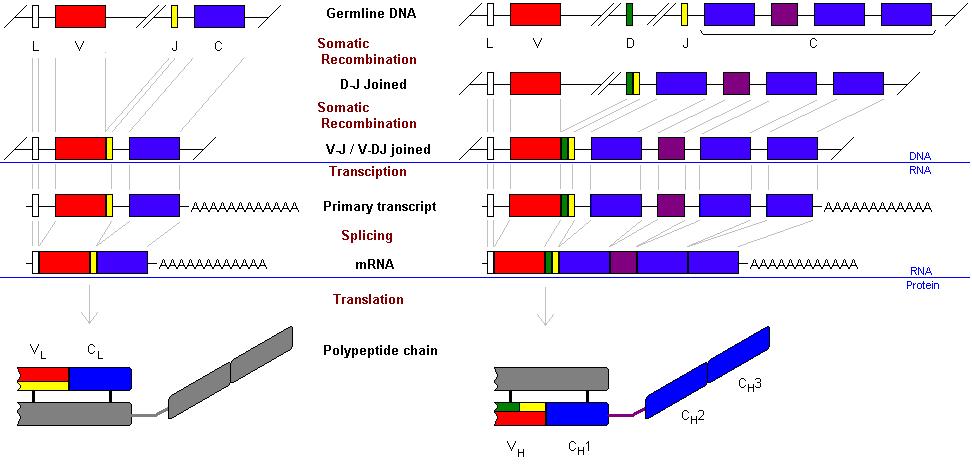
This genetic rearrangement takes place in
several steps. Firstly the heavy chain. The variable region of an
immunoglobulin molecule is made up of the variable regions of the heavy and
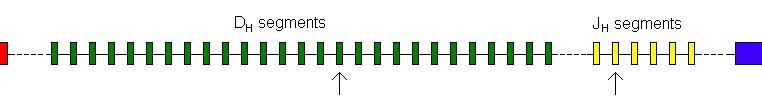
light chain. The heavy chain variable region is made from three segments. These
three are know as the Variable segment (VH), the Diversity segment
(DH) and the Joining segment (JH). The native genome has
multiple copies of each of these exons.

There are 65 different variable segments, 27
different diversity segments and 6 different joining segment. Downstream from
these segments is the coding for the conserved region of the heavy chain.
The first step in gene rearrangement is for
one of the D segments to be joined to one of the J segments. This all happens
at the DNA level under the control of the RAG1 and RAG2 gene products. (Move
mouse over image).
Any J segment can join to any D segment.
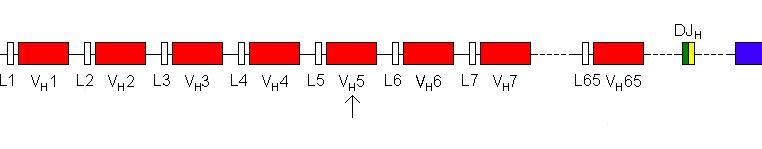
In the same way the DJ segment then joins to
one of the 65 V segments. (Move mouse over image).
Again, any V segment can join to any D
segment.
The gene is then transcribed into RNA. The
gap between the J segment and the beginning of the conserved region is removed
by splicing (as are the introns within the conserved region). This produces an
IgM heavy chain. (The L segment codes for a leader part of the mRNA which is
not translated and thus does not code for part of the peptide.)
The process is essentially the same for the
light chains with two differences. Firstly there are two genes for the light
chain – k and l (either one can be used). Secondly there are
no D segments in the light chain, only J segments.
The overall process is summarised below.
The k light chain gene contains 40 variable segments and 5 J segments.
The l gene 30 V and 4 joining segments.
Hence there are 40 x 5 = 200 different combinations for the k light chain and 30 x 4 = 120 for the l light chain. Thus by the random arrangement
of J with D segments alone, there are 320 different light chains that are
possible. Similarly with the heavy chain: 65 V segments x 27 D segments x 6 J segments
= 10530 different heavy chains. If any heavy chain can combine with any light
chain then that would result in 10530 x 320 = 3369600 (3.4 million) different
antibodies that B-lymphocytes could produce.
3.4 million (3x106) is quite a
large number but it is a long way from 1011. The answer as to how
this much diversity is produced lies in the means by which these different
segments are joined together. It is not simply a means of excising the
intervening sequences and joining together the segments but two processes occur
within the joints to vastly increase the diversity of these peptides. This is
referred to as joint diversity.
Joint Diversity: N- and P- nucleotides
N-nucleotides are so-named because they are not
coded for by the gene and P-nucleotides are palindromic sequences that
are added at the joints between segments.
P-Nucleotides
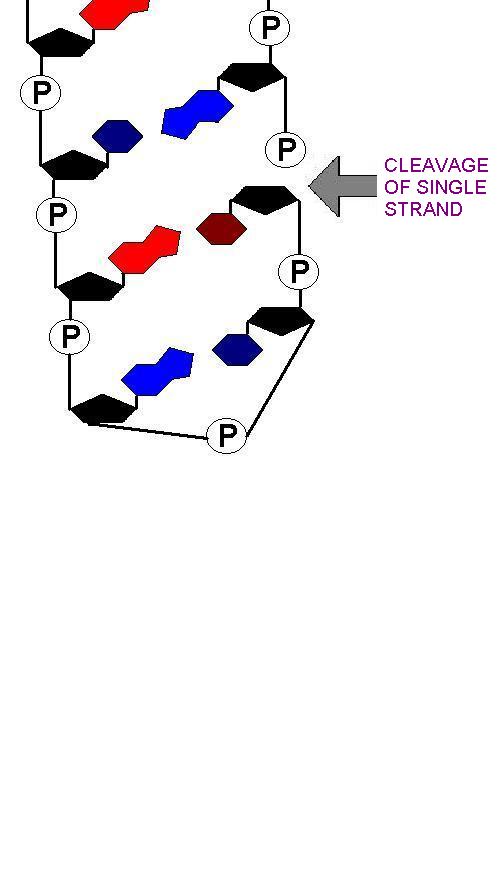
When Rag-1 and Rag-2 cleave the ends of a D segment and a J segment they form a hairpin loop in the DNA. A hairpin loop is formed when the two anti-parallel strands of DNA are joined together:
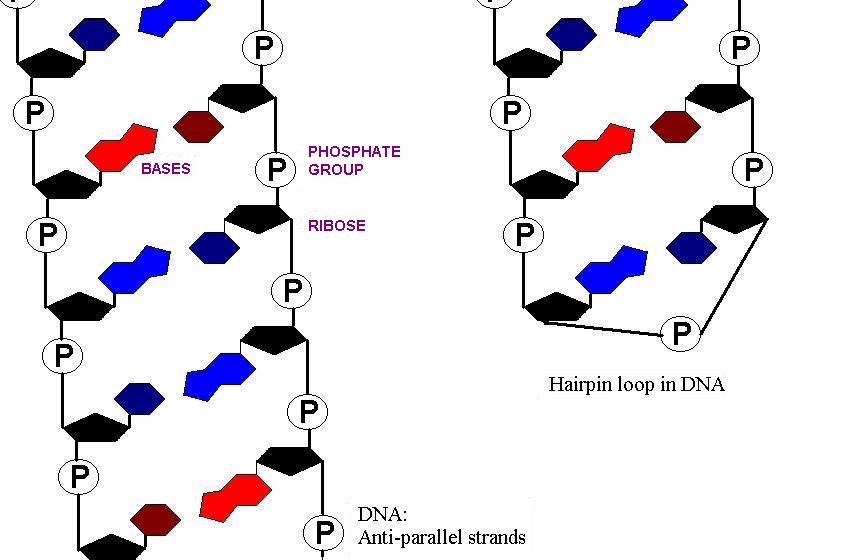
Then, single stranded cleavage of one of the strands of DNA two or three bases from the loop takes place: (Move mouse over image)
This will, by definition, result in a palindromic sequence:
Native DNA:
5' - CTGAAGTTC - 3'
3' - GACTTCAAG - 5'
Hairpin loop unwound:
5' - CTGAAGTTCGAAC - 3'
3' - GACTT - 5'
The seqeunce GTTCGAAC is palindromic as the opposite strand (running in the opposite direction is the same): 3' - CAAGCTTG- 5' is 5'-GTTCGAAC-3'
The unwound hairpins thus make 'sticky-ends' of the DNA which can be brought together:
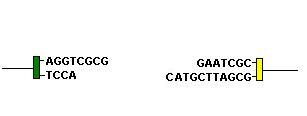
N-Nucleotides
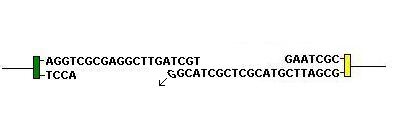
In heavy chain rearrangement and sometimes in light chain rearrangement, another enzyme TdT (Terminal deoxynucleotidyl Transferase) is active. It acts to add random nucleotides to the long single stranded end. Anything up to 20 nucleotides may be added.

The 'sticky ends' are then brought together and the gaps filled in by DNA repair enzymes: (Move mouse over image)
By these mechanisms at D-J and V-D joints in heavy chains and at V-J joints in light chains, massive diversity is produced. The number of nucleotides added is random. If this is not a multiple of three it will produce a frame shift. Frame shifts produce non-functioning peptides and therefore only 1 in every 3 rearrangement results if a functioning peptude and so this is a very wasteful process.
Somatic Hypermutation
All of these processes occur in the immature B cell as they are necessary to produce the B-cell receptor molecule. One final process that occurs in mature B lymphocytes is somatic hypermutation. Somatic hypermutation is the mutation of the immunoglobulin gene in a mature B-lymphocyte. This occurs in the V regions of the immunoglobulin gene where point mutations are made.
The major signal for B-cell proliferation is binding of antigen. If hypermutation produces an immunoglobulin molecule that has a higher affinity for the antigen than the original molecule, this B cell is then selected in a kind of 'micro-evolution' because the new immunoglobulin molecule will bind the antigen more tightly. This process produces more effective antibodies that have better affinity for the antigens.
Isotype
Switching
The different classes of antibody are referred to as isotypes. Na´ve B-lymphocytes express IgM (and IgD). In the event of activation they divide and differentiate. Some of the daughter cells become plasma cells, secreting large amounts of IgM. Other daughter cells become memory cells and undergo isotype switching, producing either IgG or IgE or IgA. Isotype switching is also done at the DNA level and thus is irreversible.
The heavy chain genes are laid out as follows: the 65 V
segments are followed by the 27 D segments and then by the 6 J segments.
Downstream from the J segments are all the conserved segments. First the m and then
the d segments. An immature B cell undergoes the gene
rearrangements described above to form the genetic code for the variable part
of the immunoglobulin molecule. This is then fixed for the life of the B cell.
Whatever antigen specificity it has is maintained, regardless of which isotype
of immunoglobulin it produces. This is because the variable part is transcribed
into mRNA along with whichever conserved segment it is expressing.
In the na´ve cell the m and d segments are both transcribed into mRNA. One of these is removed by alternate splicing. Thus translating the mRNA produces either an IgM molecule or an IgD molecule. Since it is a random process that determines which conserved segment is removed, IgM and IgD are co-expressed by the same cell. Further downstream are the coding regions for g3, g1, a1, g2, g4, then e and finally a2. In order for these to be expressed the intervening sequences of DNA are removed. For example, to form an IgA1 antibody, the cell would excise all of the segments between the VDJ segment and the a1 segment. Hence, the m, d, g3 and g1 segments are all lost from the genome.